Transporting Biological Agents
 If moving biological agents from a site/place of work, consideration must be given to the Dangerous Goods Modal Transport Regulations - the modes being air, road, sea or rail. Dangerous goods are substances and articles which have been identified as hazardous for transport and present a risk to people, property and the environment. The Health and Safety Authority enforces legislation covering the carriage of dangerous goods by road nationally (the Department of Transport, Tourism and Sport and its relevant agencies being responsible for the other modes of transport).
If moving biological agents from a site/place of work, consideration must be given to the Dangerous Goods Modal Transport Regulations - the modes being air, road, sea or rail. Dangerous goods are substances and articles which have been identified as hazardous for transport and present a risk to people, property and the environment. The Health and Safety Authority enforces legislation covering the carriage of dangerous goods by road nationally (the Department of Transport, Tourism and Sport and its relevant agencies being responsible for the other modes of transport).
National regulations governing the transport of dangerous good by road give effect to an international agreement; European Agreement Concerning the International Carriage of Dangerous Goods by Road, commonly known as ADR. This international agreement forms the basis for national and international transport of dangerous goods in 51 countries worldwide (predominately Europe and the surrounding countries).
There are 4 steps involved in the safe transport of dangerous goods by road:
- Classification;
- Packaging;
- Labelling; and
- Requirements for carriage (driver training, vehicle safety equipment, documentation etc.).
Classification
Under ADR there are 9 specific hazard classification groups, some of which are subdivided. Biological agents which are being transported by road need first to be classified and when appropriate assigned to the relevant hazard class. Biological agents which are classed as pathogenic (as defined by ADR - see below) will be assigned to Class 6.2, infectious substance of ADR. Genetically modified microorganisms (GMMs) will generally be assigned to Class 9 if they do not meet the definition of toxic substances or of infectious substances under ADR but are capable of altering animals, plants or microbiological substances in a way not normally the result of natural reproduction.
Note: Class 6.2 of ADR does not refer specifically to biological agents but instead refers to infectious substances. Infectious substances are defined under ADR as substances which are known or are reasonably expected to carry pathogens. Pathogens in turn are defined under ADR as microorganisms (including bacteria, viruses, rickettsiae, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals. It should be noted that ADR is broader in scope than the Biological Agents Regulations as it covers microorganisms which cause disease in animals also.
Substances that do not contain infectious substances or that are unlikely to cause disease in humans or animals are not subject to ADR unless they meet the criteria for inclusion in another class e.g. flammable, toxic etc. A Dangerous Goods Safety Adviser (DGSA) may be required to assist with classification. If transporting using several different modes of transport e.g. road and air, advice from a competent person specialising in multi-modal transport may be required.
Class 6.2
Substances of class 6.2 are subdivided as follows:
I1 Infectious substances affecting humans;
I2 Infectious substances affecting animals only;
I3 Clinical waste;
I4 Biological substances.
Infectious substances that fall into Class 6.2 are divided into 2 categories (category A and B) and should be assigned as appropriate to United Nations (UN) numbers 2814, 2900, 3291 or 3373 (UN numbers are four-digit numbers that identify hazardous materials and articles, these numbers assist people involved in the transport chain - for example, in the event of a road traffic collision fire fighters will know what was being transported).
Category A: An infectious substance which is carried in a form that, when exposure to it occurs (when it is released outside of the protective packaging, resulting in physical contact with humans or animals), is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals. An indicative list of infectious substances is provided in the ADR.
Category B: An infectious substance which does not meet the criteria for inclusion in category A and such substances are assigned to UN No. 3373.
Packaging
Packaging of infectious substances must be designed to minimise the potential for damage during transport and also to ensure the integrity of the materials being transported. Each hazard classification group has particular specifications for packaging, labelling and transport which are set out in modal packing instructions.
Typically packaging used for the containment of dangerous goods are UN approved packaging systems and are identified by the symbol:
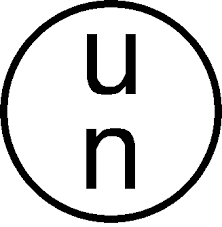
Labelling
Packages containing infectious substances category A and clinical waste must be labelled with the following hazard class 6.2 label:

Package must also be marked with the appropriate UN number and for certain modes of transport the "proper shipping name" must also be clearly legible.
UN Number and Proper Shipping Names
UN No. 2814 Infectious Substance, Affecting Humans
UN No. 2900 Infectious Substance, Affecting Animals, only
UN No. 3291 Clinical Waste, Unspecified N.O.S. or (Bio)Medical Waste, N.O.S. or Regulated Medical Waste, N.O.S.
For packages containing category B infectious substances, the package must be marked with the following:

BIOLOGICAL SUBSTANCE, CATEGORY B
For further information, click here.