Registration
The Registration Dossier
The Registration Dossier consists of two main components
- The Technical Dossier
- The Chemical Safety Report
REACH Annexes VI – XI specify the information that is required to be submitted for the purposes of Registration as part of the ‘technical dossier’. Annex VI gives details of the minimum information required for a registration while Annexes VII to X detail the additional information requirements depending on tonnage bands. A summary of the information requirements for the different tonnage bands is given below.
The Technical Dossier| Substance Criteria | Standard Information Requirements |
| Non phase-in substances at ≥ 1 tonne per year | Annex VII |
| Phase-in substances at ≥ 1 tonne per year meeting one or both of the criteria specified in Annex III | Annex VII |
| Phase-in substances at ≥ 1 tonne per year which do not meet either criteria specified in Annex III | Annex VII, section 7 (physico-chemical properties of the substance) |
| Substances at ≥ 10tonnes per year | Annexes VII and VIII |
| Substances at ≥ 100 tonnes per year | Annexes VII and VIII data and testing proposals for information specified in Annex IX |
| Substances ≥ 1000 tonnes per year | Annexes VIII and VIII data and testing proposals for information specified in Annexes IX and X |
Further information on what data is required to complete a registration dossier under REACH can be found in Chapter 5 of the ECHA. Guidance in a Nutshell on Registration and in Section 1.8 of the Technical Guidance on Registration.
Chemical Safety Assessment
A Chemical Safety Assessment (CSA) is required for all substances manufactured or imported in quantities of 10 Tonnes or more per year with the exception of intermediates used under strictly controlled conditions and for substances in mixtures in a concentration below the concentration limits in Directive 1999/45/EC and CLP. A CSA in REACH is the instrument which collects and collates all the available information on a substance and aims to assess the hazards and risks to human health and the environment and to determine how to control these risks by applying suitable risk management measures. The assessment takes a repetitious approach as illustrated in the diagram below. ECHA have produced guidance detailing the process of the Chemical Safety Assessment. This guide can be downloaded from the following link
Elements of a Chemical Safety Assessment
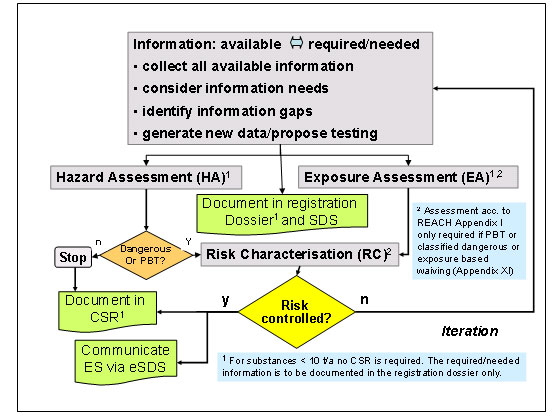
Chemical Safety Report
The Chemical Safety Assessment is documented in the Chemical Safety Report (CSR) which is submitted by the registrant together with the technical dossier as part of the Registration process.
Further Information on these communication tools and their application can be found in the section on Information in the Supply Chain.
[back to top]
Data sharing
Since one of the main principles of REACH is to avoid animal testing it is envisaged that much of the testing data required for a registration dossier will be shared among members of a Substance Information Exchange Forum (SIEF) so as to avoid the need for any new test data to be generated where this data already exists.
The members of a SIEF for a particular substance will assess and share available data, and prepare the common parts of the dossier together. New studies shall be conducted “only as a last resort”. Where new data is required to be generated for a particular property; a testing proposal must be submitted to ECHA prior to any testing. New tests should be carried out in accordance with standard EU test methods or equivalent guidelines such as those from the Organisation for Economic Co-operation and Development (OECD) and in accordance with the Test Methods Regulation. Further information can be found in the ECHA guidance on Data Sharing.
Joint Submission
Registrants of the same substance are not required to submit the registration dossiers individually. When potential registrants in a SIEF have assessed and shared the available data, the Lead Registrant (LR) prepares the common parts of their registration dossier, the so-called joint submission (JS). The other parts of the registration dossier have to be submitted separately by the members of the SIEF. A joint submission dossier consists of:
- Study Summaries/ Robust study summaries
- Classification and labelling
- Testing Proposal for additional studies, where applicable
Registrants may also decide to jointly submit the following:
- Guidance on safe use of the substance
- Chemical Safety Report when required
- Whether the relevant information has been reviewed by an assessor (on a voluntary basis)
The company acting as the Lead Registrant will submit the joint submission (JS). The JS is made on behalf of all the registrants further to that subsequent Registrants must submit company specific information separately.
Dossier Submission
A registration dossier must be prepared and submitted using UCLID 5 and REACH IT. Dossiers must be prepared in IUCLID 5 format and subsequently submitted to ECHA electronically via REACH IT. For information on IUCLID 5 visit the Software tools section of the ECHA website. REACH IT is the central IT system providing support to REACH and is managed by ECHA. The REACH IT portal is accessible from the REACH IT Homepage of the ECHA website.
Data Submission Manual 5: How to Complete a Technical Dossier for Registrations and PPORD Notifications (published by ECHA) aims to assist registrants with their dossier submissions to ECHA.
Completeness Check
Further to the submission of a dossier, ECHA undertakes a ‘completeness check’ to ensure that all elements required by REACH have been provided by the Registrant. Where data is missing, the registrant is informed and must resubmit the dossier (including the missing data). Once ECHA considers the dossier complete and the appropriate fee has been received ECHA issues a registration number. The dossier processing web page (on the ECHA website) provides an overview of the steps performed at ECHA following receipt of a dossier, including the timeline and communication steps under REACH-IT
Data Submission Manual 4 gives details on the business rule validation in REACH IT.
Fees
The Fee structure for the submission of Registrations to ECHA has been set in the Fees Regulation. Reduced fees apply to joint submissions and also to SME's.
[back to top]